Novavax Uk Trial Results - Novavax skyrockets 21% after saying its COVID-19 vaccine ... : Novavax is still signing up volunteers for a.
Novavax Uk Trial Results - Novavax skyrockets 21% after saying its COVID-19 vaccine ... : Novavax is still signing up volunteers for a.. While the novavax vaccine study is still ongoing, initial results show it is nearly 96% effective against the older coronavirus and nearly 86% effective against the new variant circulating in the uk. The trial results could soon put the vaccine on a path to authorization in multiple countries. Half the cases of covid in the trial were identified as caused by the variant. Novavax said the trial, which enrolled 15,000 people aged 18 to 84, is expected to be used to apply for regulatory review in britain, the european the study took place as the more highly transmissible uk variant was circulating, and the preliminary analysis suggests the vaccine was 85.6 per cent effective. First, however, the study results must be.
Professor paul heath, chief investigator of the uk novavax trial, said the results were very important, particularly in regards to the efficacy against the uk variant. Novavax received $1.6 billion from the u.s. Novavax will have a conference call to further discuss these. Large phase iii trial in the the british study is now set to serve as the basis for approval procedures in the uk, the eu and other countries. Novavax also announced successful results of its phase 2b study conducted in south africa.

Get the latest novavax earnings report, revenues as well as upcoming nvax earnings dates, historical financial reports, news the latest earnings per stock, revenues and financial reports for novavax inc (nvax).
The novavax coronavirus vaccine could submit its results to the uk regulator within two weeks, says the clinical trial chief, with supplies set to be available to early results show the jab is 89.3 effective against the virus and can fight off the kent strain, in a glimmer of hope that the uk will add a fourth. Our medicines regulator will now assess the vaccine its chairman clive dix saying in a statement: Novavax is also conducting a large federally funded trial in the u.s. Novavax will have a conference call to further discuss these results at 5 p.m. Large phase iii trial in the the british study is now set to serve as the basis for approval procedures in the uk, the eu and other countries. Our medicines regulator will now assess the vaccine, which will be made in teesside. First, however, the study results must be. The nurse i talked to said she thought the uk results will come in february some time. This kind of study can be valuable for doctors by determining whether it is safe to give patients two vaccines at the same time. Novavax is testing a new coronavirus vaccine on human subjects for the first time, launching a phase one clinical trial with a massive grant from an agency we look forward to sharing the clinical results in july and, if promising, quickly initiating the phase 2 portion of the trial. Novavax said on monday it has started the phase 1 clinical trial of a novel coronavirus vaccine candidate and has enrolled the trial's first participants, with preliminary results slated for july. If the results are positive, the study could lead to government authorization of the use of novavax's shot in the u.k. Half the cases of covid in the trial were identified as caused by the variant.
As i previously mentioned.trial participants have begun divulging their trial vaccination status on twitter. Novavax is also conducting a large federally funded trial in the u.s. The novavax trial in south africa will be funded by the bill & melinda gates foundation, which is providing a $15 million grant, and the coalition for epidemic preparedness innovations (cepi), which is providing funds to manufacture doses of the vaccine needed for the trial, according to the statement. While the novavax vaccine study is still ongoing, initial results show it is nearly 96% effective against the older coronavirus and nearly 86% effective against the new variant circulating in the uk. Novavax will have a conference call to further discuss these results at 5 p.m.
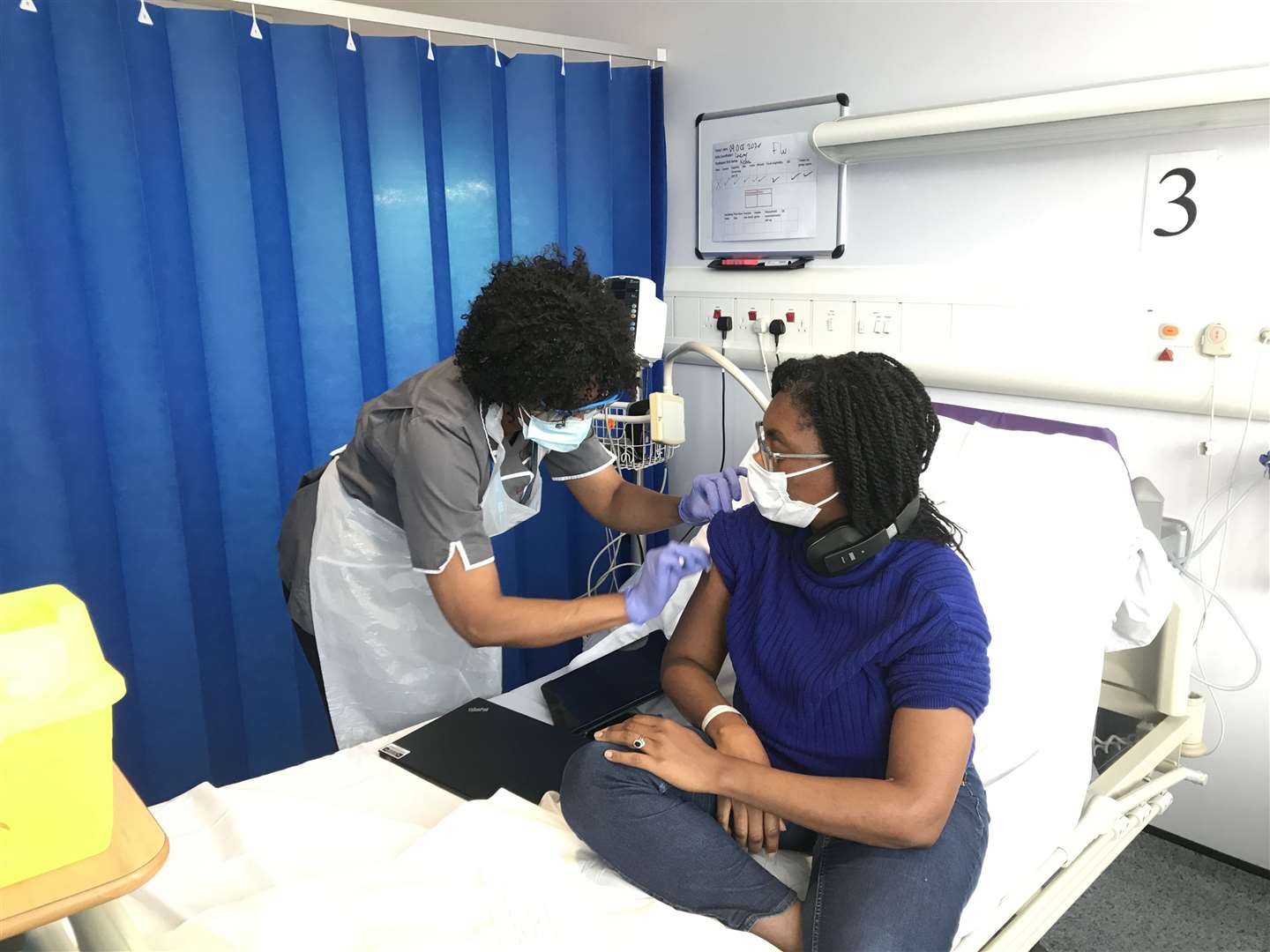
The novavax jab's 89.3 per cent efficacy in uk trials was called spectacular by expert clive dix.
Novavax said the trial, which enrolled 15,000 people aged 18 to 84, is expected to be used to apply for regulatory review in britain, the european the study took place as the more highly transmissible uk variant was circulating, and the preliminary analysis suggests the vaccine was 85.6 per cent effective. This kind of study can be valuable for doctors by determining whether it is safe to give patients two vaccines at the same time. Our medicines regulator will now assess the vaccine its chairman clive dix saying in a statement: The promising results come after the jab was trialled in the uk and south africa. And other countries, he said, without naming them. Novavax's uk trial will distinguish itself from competitors because up to 400 of the volunteers will also receive an approved flu shot. The nurse i talked to said she thought the uk results will come in february some time. If the results are positive, the study could lead to government authorization of the use of novavax's shot in the u.k. In a trial in south africa, the vaccine was far less effective, possibly because of a coronavirus variant that has emerged there. The pharmaceutical firm said it expects to use the results to apply for a regulatory review in britain, the european union and other. Thank you to all the volunteers who made these results possible. The trial results could soon put the vaccine on a path to authorization in multiple countries. Professor paul heath, chief investigator of the uk novavax trial, said the results were very important, particularly in regards to the efficacy against the uk variant.
Large phase iii trial in the the british study is now set to serve as the basis for approval procedures in the uk, the eu and other countries. Novavax will have a conference call to further discuss these results at 5 p.m. Novavax said on monday it has started the phase 1 clinical trial of a novel coronavirus vaccine candidate and has enrolled the trial's first participants, with preliminary results slated for july. The pharmaceutical firm said it expects to use the results to apply for a regulatory review in britain, the european union and other. First, however, the study results must be.

Novavax will have a conference call to further discuss these results at 5 p.m.
Good news that the @novavax vaccine has proved effective in uk trials. The pharmaceutical firm said it expects to use the results to apply for a regulatory review in britain, the european union and other. First, however, the study results must be. If the results are positive, the study could lead to government authorization of the use of novavax's shot in the u.k. Thank you to all the volunteers who made these results possible. Novavax also announced successful results of its phase 2b study conducted in south africa. Thank you to all the volunteers who made these results possible. The uk trial, which enrolled 15,000 people aged 18 to 84, is expected to be used to apply for use in britain, the john moore, a professor of microbiology and immunology at weill cornell medical college in new york, said the novavax uk data are essentially the same as results from pfizer and moderna. The novavax coronavirus vaccine could submit its results to the uk regulator within two weeks, says the clinical trial chief, with supplies set to be available to early results show the jab is 89.3 effective against the virus and can fight off the kent strain, in a glimmer of hope that the uk will add a fourth. The new initiative got off the. Novavax's uk trial will distinguish itself from competitors because up to 400 of the volunteers will also receive an approved flu shot. The promising results come after the jab was trialled in the uk and south africa. Novavax is also conducting a large federally funded trial in the u.s.
Komentar
Posting Komentar